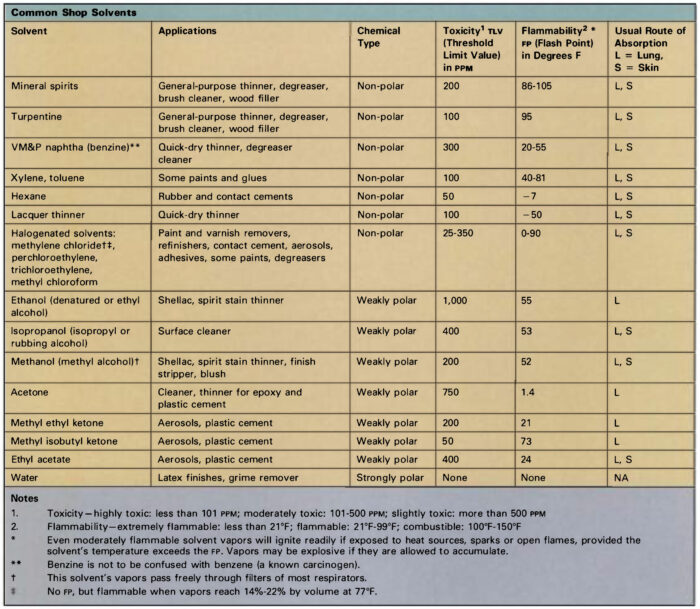
Common solvents in the woodshop
Understanding the chemicals in your shop is the first step to safe and responsible use.
The more I learn about finishing, the more I feel the need to understand exactly what is going on in my finishes. The first step to understanding a finish is usually understanding the solvent needed to work the finish. I recently found this article in our archives and thought it would help some readers. It might be older, but it’s still relatively up to date and certainly useful in my shop.
—Ben Strano
The alchemists of the Middle Ages alternated their attempts to transmute lead into gold with the search for a universal solvent—a liquid capable of dissolving all materials (had they succeeded, I wonder how they’d have packaged their discovery). Six hundred years later, I’m still following in the alchemists’ footsteps. As a chemist, I’m frequently asked to dilute a dish of gooey stuff or to dissolve some residue without damaging the underlying material. Woodworkers often face similar problems. Luckily, if you know a little chemistry, you can select a solvent that not only does the job, but also poses the least health and safety hazards.
Despite the rising popularity of water-based finishes, sooner or later you will need to use another solvent besides water. Solvents work in one of two ways. Inert solvents, like mineral spirits and lacquer thinner, reduce the viscosity of finishes and allow deeper penetration, more even application, and faster drying. They don’t alter the composition of the oils or resins used in the finish. In contrast, reactive solvents attack the chemical structure of the materials they dissolve; for example, when methylene chloride is applied to paint, it becomes a paint stripper.
Many petroleum-based liquids can be used as thinners for brush-on varnishes and paints, and even though most of these organic solvents perform similarly, their toxicities vary widely. The least hazardous choice for brush-on varnish and paint thinner is mineral spirits. Turpentine also has low toxicity, but it’s more likely to cause allergic skin reactions. In fact, prolonged or repeated skin exposure to most solvents (except water) can cause skin irritation. This occurs because many solvents, like acetone, ethanol, and ketones, extract natural oils from skin layers, which results in severe chapping. If you need a thinner that dries quickly, naphtha is safer than most other fast-evaporating solvents, but be aware that quick-dry solvents all pose substantial fire hazards. Woodworkers are usually surprised to learn that the most flammable shop solvent is acetone—a liquid that’s often handled with very little caution.
What’s in a label: Common solvents in the woodshop
Aliphatic hydrocarbons: Also known as “paraffins,” these petroleum derivatives consist of chains of carbon and hydrogen atoms. Gaseous forms include methane, butane, and propane; molecules containing five or more carbon atoms are liquid at room temperature. Pentane, hexane, heptane, and octane are major constituents of gasoline, kerosene, mineral spirits, and VM&P (varnish makers’ and painters’) naphtha. Hexane is widely used in rubber-based liquids such as contact cement and rubber cement. Isobutane and propane serve as propellants in some spray cans.
Aliphatic solvents are generally less toxic than other classes of organic liquids, though they are not risk-free. Common symptoms resulting from excessive exposure include skin and respiratory irritation and central nervous system (CNS) depression.
Aromatic hydrocarbons: These compounds are ring-shaped molecules distilled from coal tar. These liquids are powerful solvents, but their use is limited by low flash-points, high volatility, and high toxicity. Three compounds are common: Toluene (toluol) and xylene (xylol) are often added to aliphatic solvents to increase their effectiveness. Benzene is not used in most areas, owing to its high toxicity and carcinogenic properties, but it is commonly present in small amounts as a contaminant in commercial-grade solvents. Benzene is commonly confused with benzine, an alternate name for VM&P naphtha, a variety of mineral spirits.
Alcohols: Denatured ethyl alcohol (ethanol) is widely used as a solvent for shellac, and consists of grain alcohol made poisonous to drink by the addition of methyl alcohol or some other toxic liquid. Methyl alcohol (methanol, “wood alcohol”) is used in lacquer thinner, paint remover, shellac, and aniline-based wood stains. Methyl alcohol can be absorbed through the skin and its vapors are much more toxic than those of denatured alcohol, so the latter product should be employed for general shop use.
Ketones and esters: This group includes a number of compounds which contain oxygen as well as carbon and hydrogen. Ethyl acetate, butyl acetate, and amyl acetate are esters used in nitrocellulose lacquer. Common ketones include acetone, methyl ethyl ketone, and methyl isobutyl ketone. Esters and ketones typically have strong odors and high flammability. They are particularly likely to irritate the skin because of their ability to dissolve natural oils, and they may produce respiratory irritation and symptoms of CNS depression. A ketone derivative, methyl ethyl ketone peroxide, is used as a catalyst for polyester resins. This strong oxidizing agent will cause serious damage to the skin and eyes, and demands careful handling.
Glycol ethers: These are another type of oxygenated organic compound used in solvents, most commonly in slow drying lacquer. Glycol ethers are highly toxic, and can cause liver, kidney, and CNS damage. In addition, they may adversely affect reproductive organs, causing birth defects and miscarriages.
Mineral spirits (VM&P naphtha, white spirits): These are distilled from petroleum, and consist mostly of the aliphatic hydrocarbons hexane, heptane, and octane. Composition varies according to the source of the crude oil and manufacturing differences from batch to batch, and chemical analysis reveals the presence of as many as 100 separate compounds in some samples. Mineral spirits are grouped into three categories:
- Odorless: Low-boiling-point ( 140°-180ºF) “odorless” spirits consist mostly of aliphatic compounds with fast evaporation rates and relatively weak solvency.
- Low odor: Medium- boiling-point (200° to 300°F) “low odor” spirits are predominantly heptane and octane fortified with small amounts of xylene and toluene.
- Regular odor: High boiling point (300° to 400° F) “low odor” mineral spirits comprise about 75% of all solvents used in the paint industry. They consist of 15% to 25% aromatic hydrocarbons.
Mineral spirits are less toxic than most other solvents, but vapors can cause skin and respiratory irritation and CNS depression. Toxicity increases in proportion to the aromatic hydrocarbon content, so odorless spirits are best for general use.
Turpentine: This is produced by steam distillation of pine gum, and consists mostly of carbon-ring compounds called turpenes. Pine gum contains about 68% solid rosin, 20% turpentine, and 12% water. Turpentine has a strong, characteristic odor, but its physical properties are very similar to mineral spirits, which has largely replaced it as a solvent. Turpentine is more chemically reactive, and will discolor upon long exposure to light or to air. Its vapors can cause respiratory irritation as well as dizziness, headache, and other signs of CNS depression. It is a strong skin irritant, and can cause severe allergic reactions after repeated contact.
Lacquer thinner: Lacquers are usually made by dissolving a cellulose derivative in a suitable solvent, though modern formulations may include alkyd resin, natural rosin, or other dissolved solids. Lacquer thinner uses about 30% esters and ketones as the active solvent, diluted with aromatic and aliphatic hydrocarbons. The ester, ketone, and aromatic content makes these solvents very volatile and relatively toxic, so they should be used only when needed and not as a general substitute for mineral spirits when thinning liquids or cleaning brushes.
-George Mustoe
Excerpted from articles in Fine Woodworking #41 and #92
Disposing of solvents responsiblyAs woodworkers, we use solvents and finishing products that end up as hazardous wastes, so it’s important to keep abreast of environmentally responsible waste-disposal methods. The Code of Federal Regulations (Title 40-Protection of the Environment) defines hazardous waste and establishes reportable quantities for releases of certain chemicals. However, these laws don’t apply to most woodworkers because they use such small amounts of organic compounds, such as solvents. Nevertheless, even small quantities of hazardous wastes can contaminate the environment. A quart of stripper carelessly discarded in a stream can contaminate millions of gallons of water. But there are some safe ways to dispose of small amounts of hazardous waste, as follows:
-Jeff Jackson is an environmental engineer and part-time woodworker living in Taylors, S.C. From Fine Woodworking #92 |





















Comments
So many articles about finishes and solvents are useless to woodworkers. This one is worse than most. Who cares about the number of carbon atoms or the shape of the molecules? I aced my college chemistry classes, but I need to understand what solvents are useful for what purpose when I am finishing my work. When do I want to use solvent X, and why don’t I want to use solvent Y in that case?
Log in or create an account to post a comment.
Sign up Log in